Dear Readers, we are happy to share the most interesting legal and policy updates concerning health industry that we read today. We hope you enjoy reading it.
1. India’s central food authority (FSSAI) has extended the enforcement date for the amended labelling requirements under the Alcoholic Beverages Regulations, 2025. The earlier deadline of 1 January 2026 is now postponed to 1 July 2026. The decision follows stakeholder concerns that mid-year labelling changes would disrupt operations, cause wastage of pre-printed labels, and create additional costs, especially due to State Excise label-registration cycles.
Source: h7.cl/1kc0S
2. India is reportedly negotiating mutual recognition agreements (MRAs) with major partners such as the US, EU, UK, Singapore and ASEAN to align inspection and testing systems. The aim is to cut rejections, ease trade friction and streamline exports of basmati rice, spices, tea, coffee, marine products, fruits and vegetables, improving global competitiveness.
Source: h7.cl/1flqZ
3. India’s central food authority (FSSAI) has issued amendments to the FSS (Alcoholic Beverages) Regulations, 2018 wherein the permissible maximum level of ethyl acetate esters in alcoholic beverages has been increase from “0.2” g/l to “3.0” g/l.
Source: h7.cl/1kc16
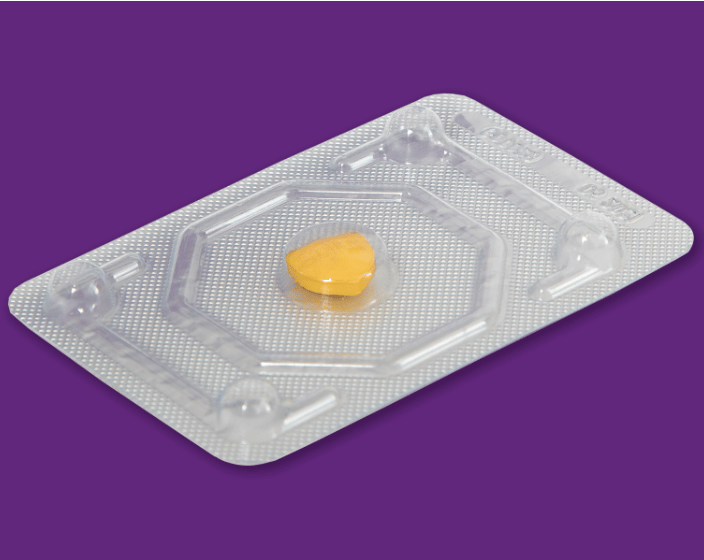
4. India’s Drugs Consultative Committee has agreed to add levonorgestrel tablets in 0.75 mg and 1.5 mg widely known as morning-after pills to Schedule K, enabling continued OTC sales. This also includes provisions for a boxed warning on packaging highlighting side effects, no protection against HIV/STIs, limits on use, and recommendations for consulting practitioners on alternatives.
Source: h7.cl/1kbV8
5. The Supreme Court of India has reportedly directed Noida District Hospital to constitute a primary medical board within two weeks to assess passive euthanasia for a 31-year-old man who has been quadriplegic and in a vegetative state for more than a decade.
Source: h7.cl/1flra